To access the training videos, please enter your code.
If you do not remember the code,
contact your Account Manager.

Our design tool allows you to build your forms using drag and drop or by programming code. You can create form rules using XML and even build custom clickable forms using JavaScript!
Designing your forms and edit checks is easy with an intuitive drag and drop user interface, which
renders them exactly as designed.
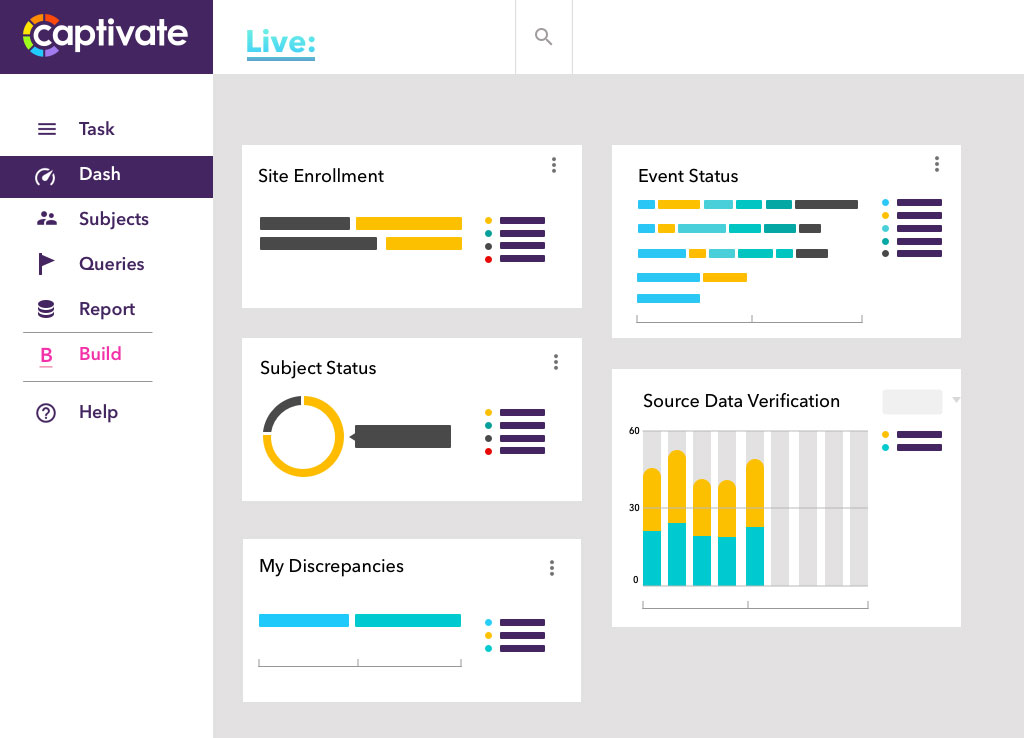
Risk Based Monitoring (RBM) supports both partial and targeted source data verification.
Study changes are both versatile and easy to manage. If a study amendment is required, a new CRF version can be assigned to specific sites or it can be made available automatically to all sites.
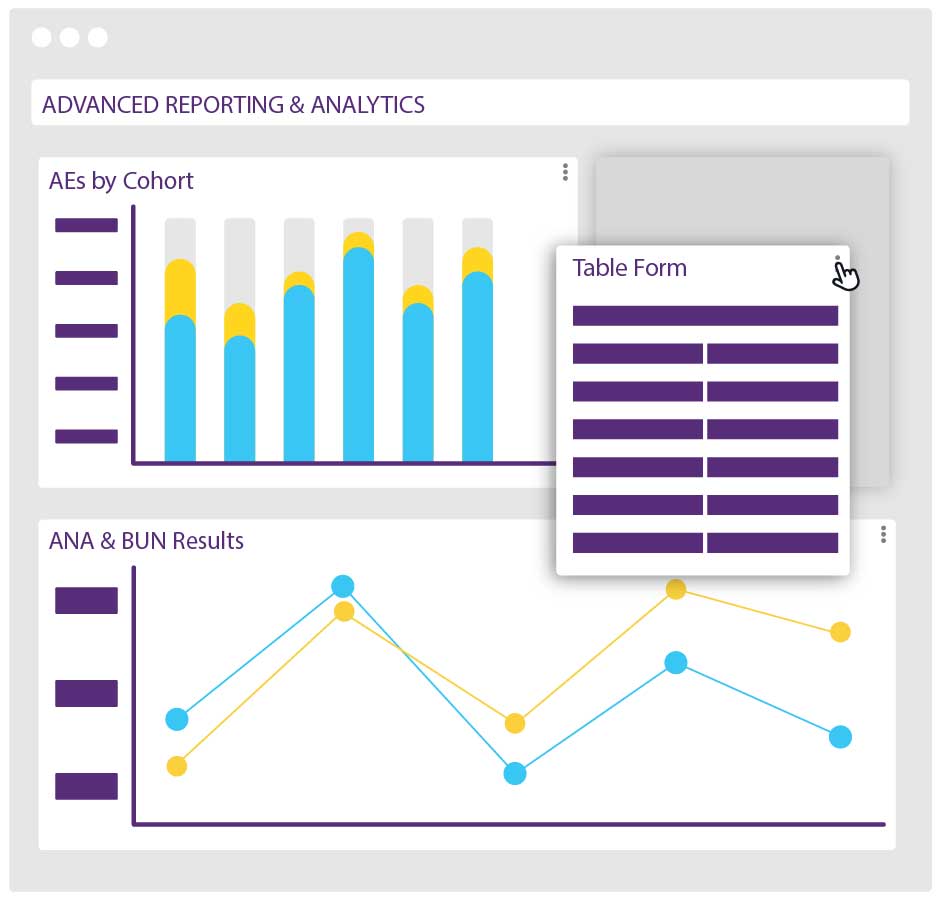
Interactive dashboards show your study progress in realtime and allow you to drill down on the data that matters.