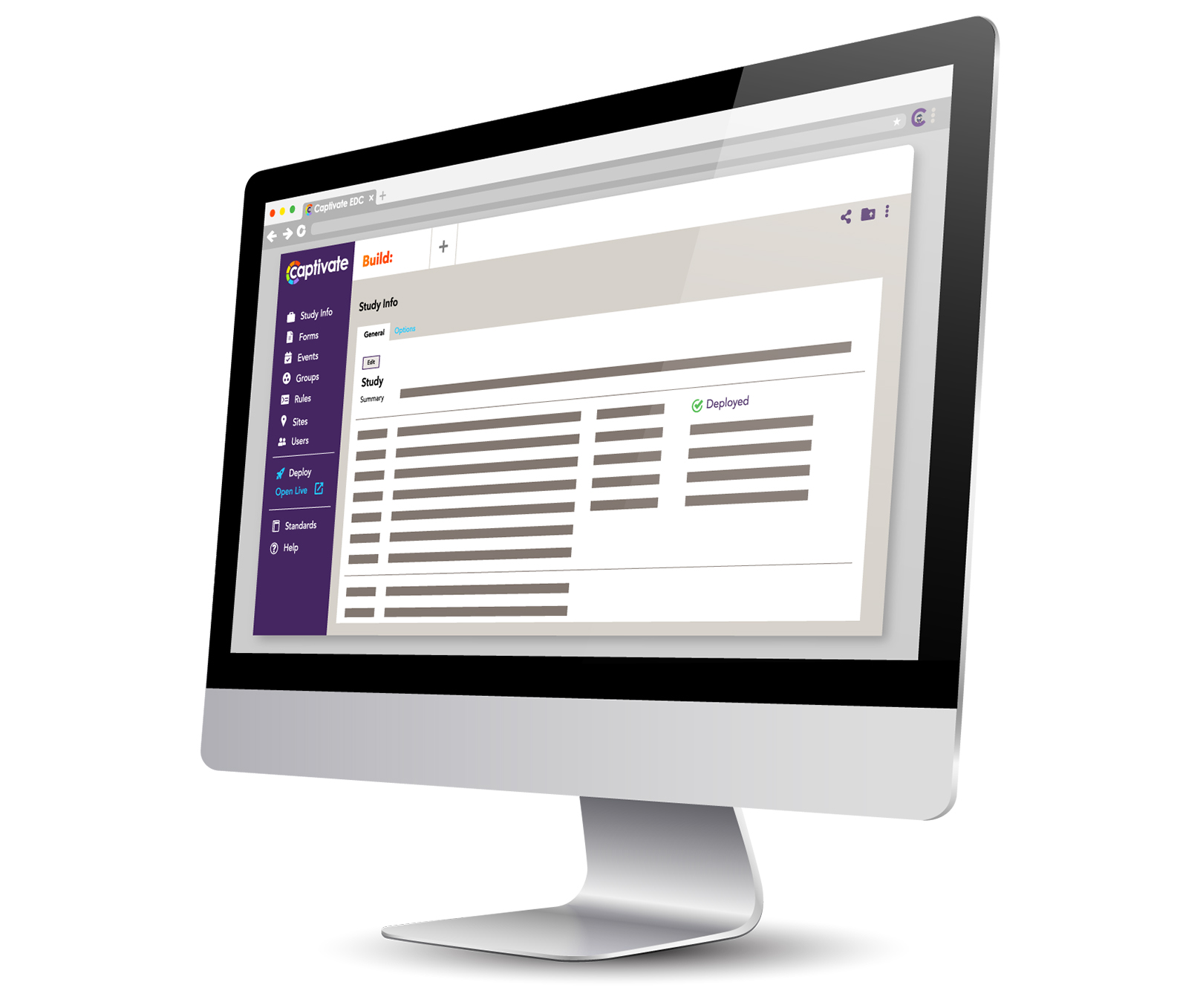
Virtual Data Capture® (VDC®) is a cutting edge suite of proprietary products designed to expedite clinical trials. The suite contains four modules that work together to accelerate researchers’ ability to plan, execute and manage Decentralized and Remote Trials. Our design tool allows you to build your forms using drag and drop or by programming code. You can create form rules using XML and even build custom clickable forms using JavaScript with our Dynamic Form Customization™ (DFT™) feature! Enabling this powerful tool allows you to utilize clickable images, Visual Analogue Scales (VAS), and other dynamic elements to better represent validated instruments.
The suite includes Captivate® electronic Patient Reported Outcomes (ePRO), electronic Consent (eConsent), electronic Clinical Outcomes Assessment (eCOA) and electronic Source records (eSource).
Here is a look at the Virtual Data Capture® (VDC®) features:
-
ePRO
Description
- Seamlessly capture data from patients
- Automatically integrate unified responses into your EDC System
- Full accessibility on desktop or mobile devices
-
eConsent
Description
- Automates patient enrollment process and onboards patients directly into Captivate® EDC
- Improves overall consent tracking management, reducing errors
- Enhances the patient experience
-
eCOA
Description
- Seamlessly capture data from observers
- Automatically integrate unified responses into Captivate®
- Easily report data on phones, tablets, computers, and other electronic devices
-
Designed for Remote Trials
Virtual Data Capture® (VDC®) was developed to help researchers manage decentralized and remote trials. While the COVID-19 Pandemic increased interest and demand for technology to conduct remote trials, ClinCapture has historically been a leader in accelerating Decentralized and Remote Clinical Trials. ClinCapture continues to enhance the features of this critical suite of products to offer the best solution for remote trials.