VDC® electronic Clinical Outcomes Assessment (eCOA) includes advanced features to seamlessly capture data from observers and automatically integrate unified responses into your EDC System.
What is eCOA?
VDC® electronic Clinical Outcomes Assessments (eCOA), allows clinicians and caregivers to use phones, tablets, computers, and other electronic devices to report data.
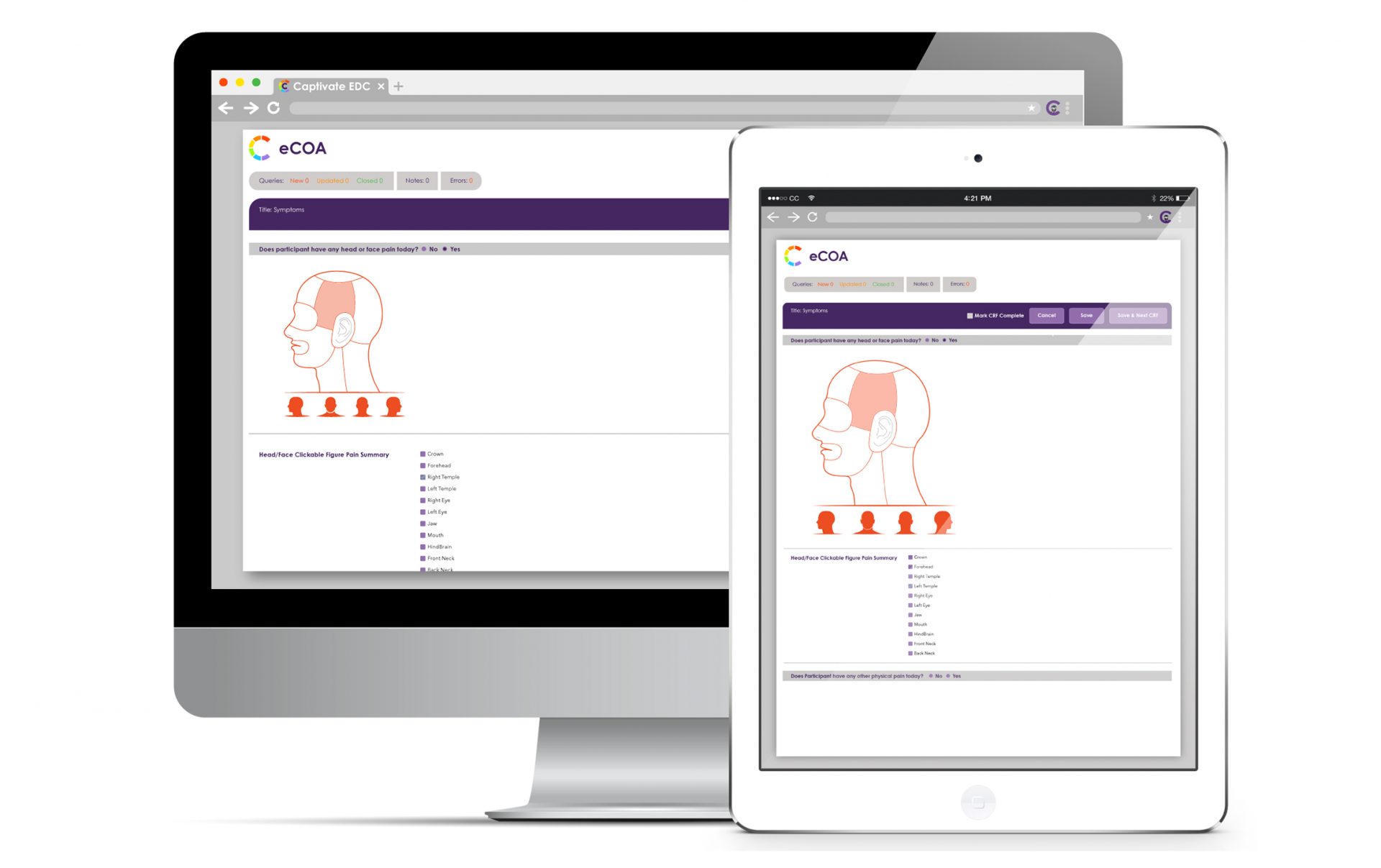
Our eCOA includes:
- Direct electronic collection of Patient Reported Outcomes, as well as Clinician Reported Outcomes and Observer Reported Outcomes (collectively eCOA), without requiring special hardware
- Timed-expiration secure token for participants to receive by email to use on any device with internet access and any HTML5 web browser, including Chrome, Firefox, Safari, Internet Explorer, and Microsoft Edge
- Text and scale-based questions, and further custom development from ClinCapture , if necessary
- Scheduled or on-demand triggers in surveys for maximum flexibility
- Configurable email messages and reminders to alert participants to expected surveys
- Selectable language for surveys/scales which allows participants to complete surveys in their native language
- Full accessibility on desktop or mobile device including Apple IOS and Android devices
- Progress tracking and completion status in EDC for site monitoring and follow up
- Unified participant responses with clinical data in EDC to simplify data review and dataset extract
- Compliance report with survey status, dates for survey sent, and reminders