10 March, 2021, by ClinCaptureUser
Captivate® was created with our customers in mind. At ClinCapture , we understand how important it is to have the ability to access trial data easily. That is why we created Captivate® Reporting, a new tool that offers pre-built industry-standard reports.
Samantha Purdie, project manager at ClinCapture and former project admin for a clinical research organization, experienced the direct impact of reporting systems for clinical trials that required either extensive implementation or difficult processes to extract data from studies.
“I never found it easy to do. It has always been a grueling process,” she said. “[In other systems,] I used to spend 15 to 20 minutes filtering data that wasn’t needed.”
Purdie explained that with prior tools, she has dealt with excess metadata that wasn’t needed for the report thus costing more of her time.
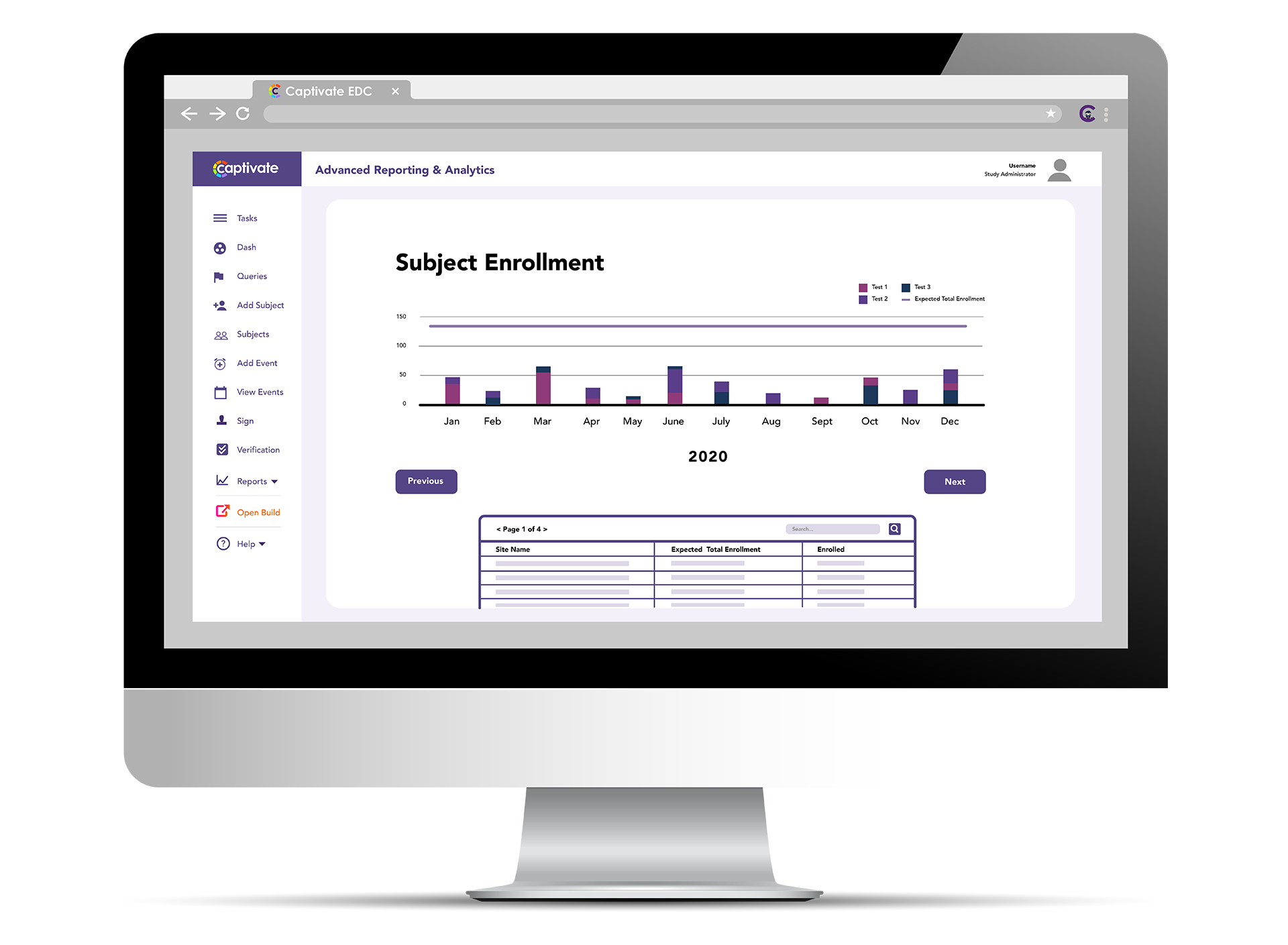
ClinCapture ’s Captivate® Reporting tool is solving that problem. Captivate® Reporting features pre-built reports that provide the data that matters for clinical trials.
- Subject Enrollment
- Query Status Report
- Subject Status
- Form Completion
- Site Activation to First Patient Visit
- Safety Dashboard
“It is a great tool,” she said. Purdie also utilizes reports featured within the Captivate® dashboard. The Captivate® dashboard features reports that provide an overview of the study in real-time.
The Captivate® dashboard features the following reports, which are available for various studies roles to see in real-time.
- Subject Status
- Events Status Summary
- Enrollment
- Event Status
- Site Enrollment
- Source Data Verification
- Signature
- My Discrepancies
- Queries
- Annotations per Case Report Forms
The reports featured in Captivate® Reporting offer configurable options to view data. For example, the safety dashboard can be separated by Serious Adverse Reaction on the site or study level, site performance can be viewed by site, and the query can be viewed by Case Report Form, study or site.
All of the study data is available in real-time for ClinCapture users and there is no need for additional software. Captivate® reporting is available directly in the application. If you are interested in viewing Captivate® Reporting, you can schedule a demo here.



